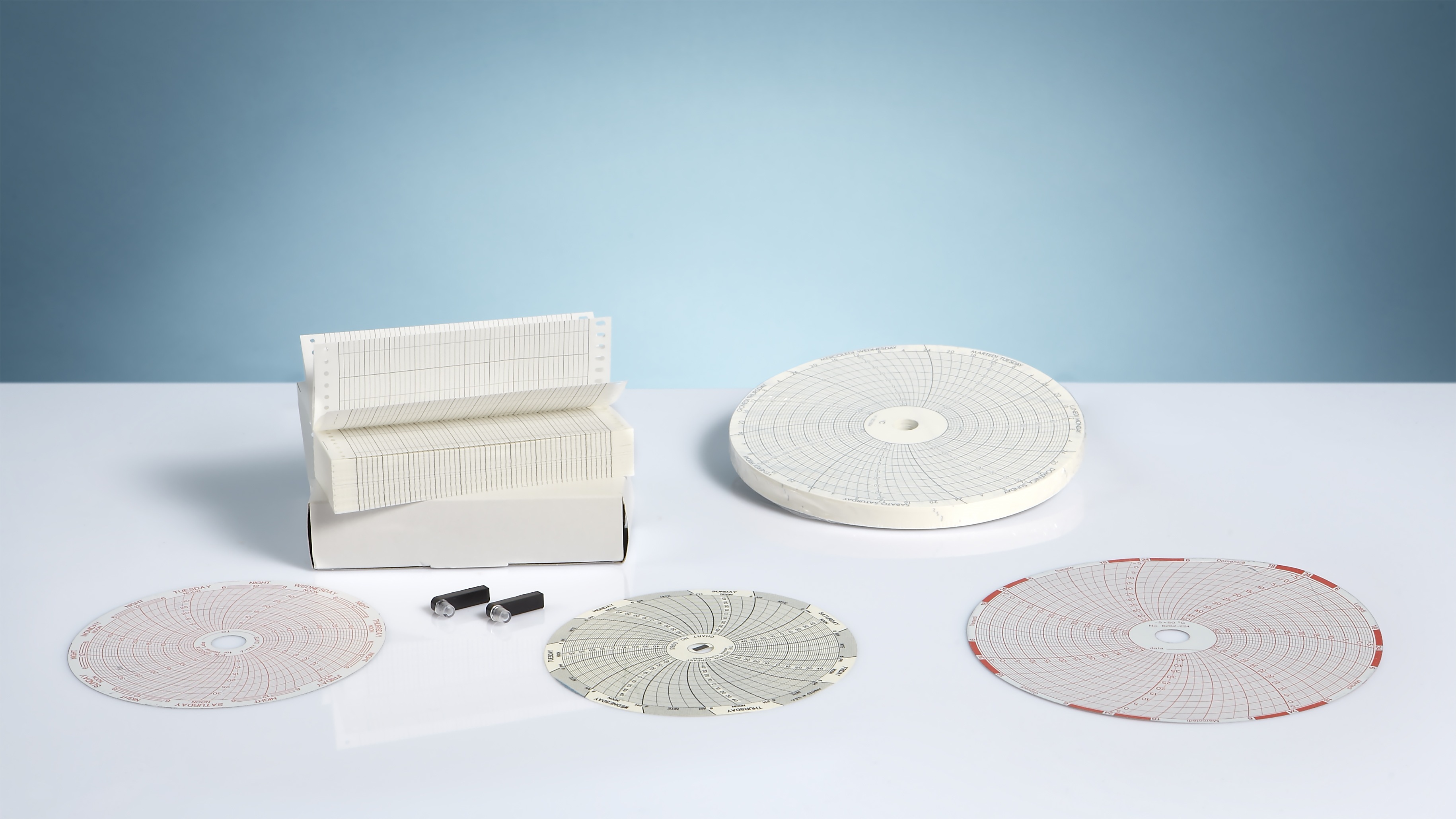
LUMED srl is a manufacturing company that has been operating in the field of medical devices for the cardiopulmonary diagnostics for over 25 years.
Our company started its activity in the field of thermal papers for medical and industrial applications and has constantly increased its products portfolio: from consumable and disposable accessories to equipment for basic cardiological and pulmonary diagnostics.

Worldwide, LUMED Srl is operating in more than 40 countries through specialized distributors and nowadays exports represent a 40% share of total sales, a number in constant and significant growth. The LUMED brand products are registered in Italy and Europe, as well as in several countries in East Asia, Middle East and South America.